3. Microfluidics for lab-on-chip biological applications
1). An on-chip microfluidic pressure regulator that facilitates reproducible loading of cells and hydrogels into microphysiological system platforms
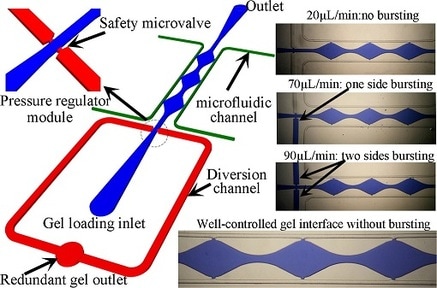
Coculturing multiple cell types together in 3-dimensional (3D) cultures better mimics the in vivo microphysiological environment, and has become widely adopted in recent years with the development of organ-on-chip systems. However, a bottleneck in set-up of these devices arises as a result of the delivery of the gel into the microfluidic chip being sensitive to pressure fluctuations, making gel confinement at a specific region challenging, especially when manual operation is performed. In this paper, we present a novel design of an on-chip regulator module with pressure-releasing safety microvalves that can facilitate stable gel delivery into designated microchannel regions while maintaining well-controlled, non-bursting gel interfaces. This pressure regulator design can be integrated into different microfluidic chip designs and is compatible with a wide variety of gel injection apparatuses operated automatically or manually at different flow rates. The sensitivity and working range of this pressure regulator can be adjusted by changing the width of its pressure releasing safety microvalve design. The effectiveness of the design is validated by its incorporation into a microfluidic platform we have developed for generating 3D vascularized micro-organs (VMOs). Reproducible gel loading is demonstrated for both an automatic syringe pump and a manually-operated micropipettor. This design allows for rapid and reproducible loading of hydrogels into microfluidic devices without the risk of bursting gel–air interfaces.
2). A hydrostatic pressure-driven passive micropump enhanced with siphon-based autofill function
Autonomous and self-powered micropumps are in critical demand for versatile cell- and tissue-based applications as well as for low-cost point-of-care testing (POCT) in microfluidics fields. The hydrostatic pressure-driven passive micropumps are simple and widely used, but they cannot maintain steady and continuous flow for long periods of time. Here, we propose a hydrostatic pressure-driven passive micropump enhanced with siphon-based autofill function, which can realize the autonomous and continuous perfusion with well-controlled steady flow over an extended time without electric power consumption. The characterization results reveal that both the cycle number in one refilling loop and the siphon diameter will affect the refilling time. Furthermore, this micropump also enables multiplexed medium delivery under either same or different flow conditions with high flexibility. The system was validated using an in vitro vasculogenesis model over the course of several days. Most importantly, the device can consistently provide steady medium perfusion for up to 5 days at a predefined hydrostatic pressure drop without the need for supplemental medium changes. We believe that this hydrostatic pressure-driven passive micropump will become a critical module for a broad range of sophisticated microfluidic operations and applications.