1. Vascularized organ-on-a-chip
1) Engineering anastomosis between living capillary networks and endothelial cell-lined microfluidic channels
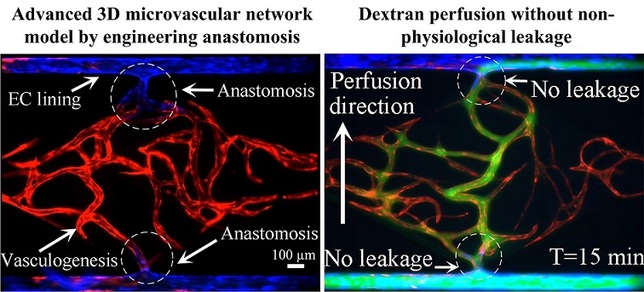
This paper reports a method for generating an intact and perfusable microvascular network that connects to microfluidic channels without appreciable leakage. This platform incorporates different stages of vascular development including vasculogenesis, endothelial cell (EC) lining, sprouting angiogenesis, and anastomosis in sequential order. After formation of a capillary network inside the tissue chamber via vasculogenesis, the adjacent microfluidic channels are lined with a monolayer of ECs, which then serve as the high-pressure input (“artery”) and low pressure output (“vein”) conduits. To promote a tight interconnection between the artery/vein and the capillary network, sprouting angiogenesis is induced, which promotes anastomosis of the vasculature inside the tissue chamber with the EC lining along the microfluidic channels. Flow of fluorescent microparticles confirms the perfusability of the lumenized microvascular network, and minimal leakage of 70 kDa FITC-dextran confirms physiologic tightness of the EC junctions and completeness of the interconnections between artery/vein and the capillary network. This versatile device design and its robust construction methodology establish a physiological transport model of interconnected perfused vessels from artery to vascularized tissue to vein. The system has utility in a wide range of organ-on-a-chip applications as it enables the physiological vascular interconnection of multiple on-chip tissue constructs that can serve as disease models for drug screening.
2) A vascularized and perfused organ-on-a-chip platform for large-scale drug screening applications
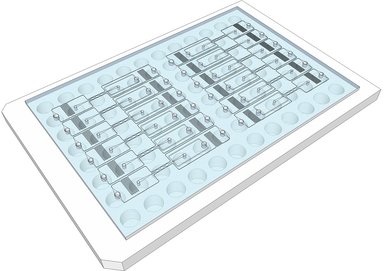
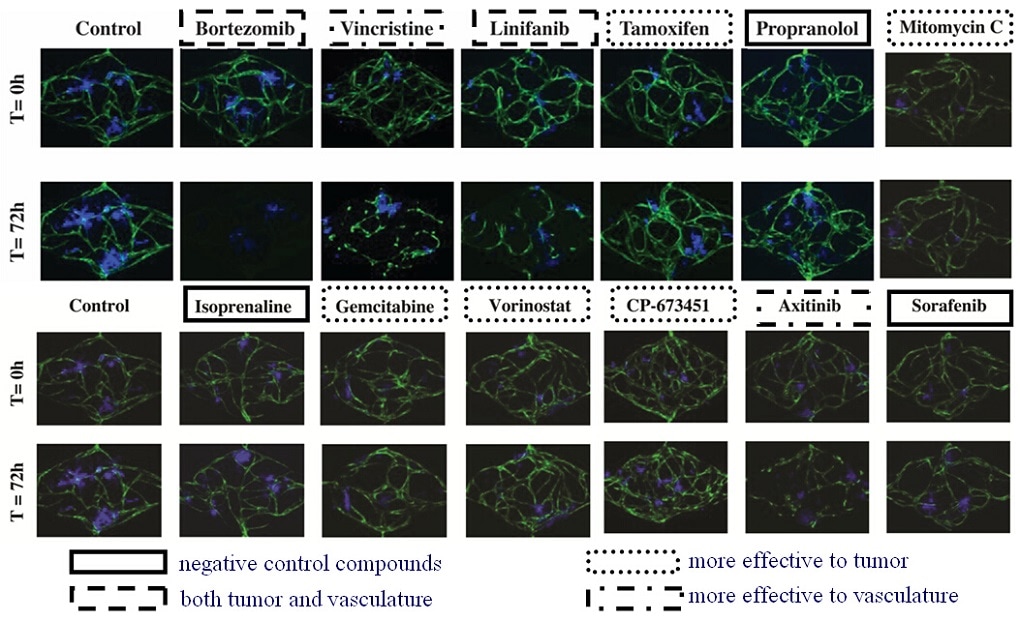
There is a growing awareness that complex 3-dimensional (3D) organs are not well represented by monolayers of a single cell type – the standard format for many drug screens. To address this deficiency, and with the goal of improving screens so that drugs with good efficacy and low toxicity can be identified, microphysiological systems (MPS) are being developed that better capture the complexity of in vivo physiology. We have previously described an organ-on-a-chip platform that incorporates perfused microvessels, such that survival of the surrounding tissue is entirely dependent on delivery of nutrients through the vessels. Here we describe an arrayed version of the platform that incorporates multiple vascularized micro-organs (VMOs) on a 96-well plate. Each VMO is independently-addressable and flow through the micro-organ is driven by hydrostatic pressure. The platform is easy to use, requires no external pumps or valves, and is highly reproducible. As a proof-of-concept we have created arrayed vascularized micro tumors (VMTs) and used these in a blinded screen to assay a small library of compounds, including FDA-approved anti-cancer drugs, and successfully identified both anti-angiogenic and anti-tumor drugs. This 3D platform is suitable for efficacy/toxicity screening against multiple tissues in a more physiological environment than previously possible.